- In this section:
- AmoyDx® HRD Focus Panel brochure
- AmoyDx® HRD Focus Panel user manual
Mutations in the homologous recombination (HR) pathway lead to homologous recombination deficiency (HRD), which is associated with several types of cancers, including breast and ovarian cancer. HRD status can be measured by “cause” through mutations in the HRR pathway (e.g., BRCA1 and BRCA2) and by the “effect” of the presence of genomic scars at a given threshold or functional assay. HRD testing can identify 30% more PARPi-effective patients than BRCA testing alone (BRCA~20% vs. HRD~50%).
The AmoyDx HRD Focus Panel provides a focused and accurate test for HRD-related BRCA1/2 mutations and Genomic Scar Scores (GSS). The panel streamlines HRD testing and helps clinicians make more informed decisions about targeted therapy for patients with HRD-positive tumours.
Single-assay solution to detect all HRD parameters

Benefits of Amoy’s HRD focus panel
- Comprehensive HRD detection – assesses both causes and effects of HRD
- Enhances precision oncology – assists in determining patient eligibility for PARPi treatment
- Advanced genomic instability assessment – utilises proprietary HANDLE technology and the Genomic Scar Score (GSS) algorithm for high-performance genomic instability detection
- Seamless laboratory integration – compatible with the AmoyDx NGS Data Analysis System (ANDAS), providing automated and secure data analysis for streamlined workflows.
- Fast and cost-effective – requires less than 1 hour of hands-on time, offering a dependable and efficient solution for evaluating PARPi-related biomarkers.
Applications of the HRD focus panel
- Homologous recombination deficiency (HRD) status assessment
- Predicting response to PARP inhibitors
- Comprehensive genomic instability evaluation for precision oncology
- Companion diagnostic for HRD-targeted therapies
Longer progression-free survival (PFS) with PARPi treatment for GSS-positive groups
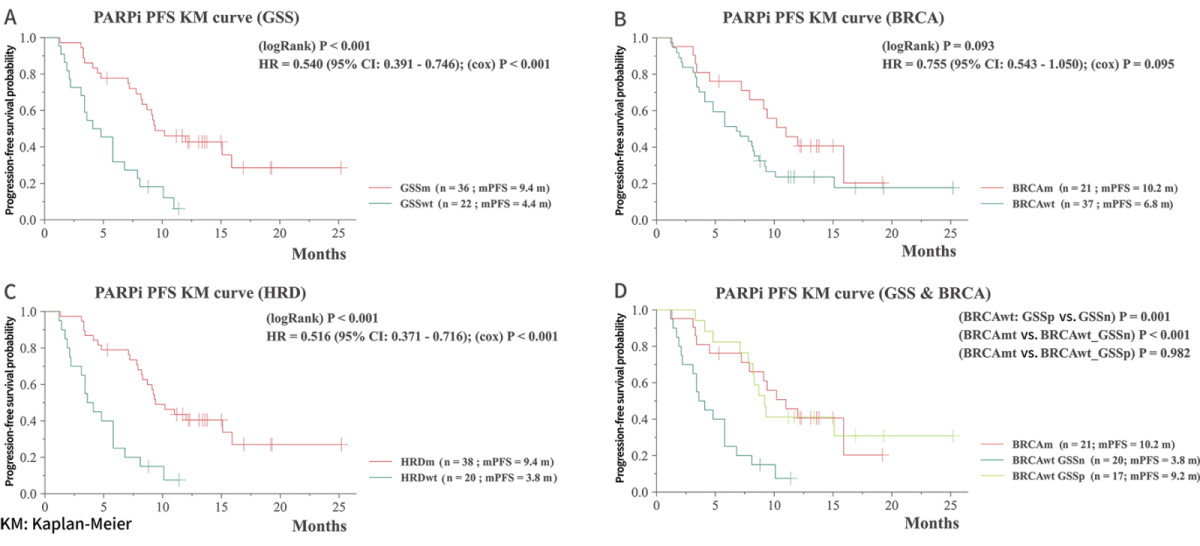
The study highlights the promising value of GSS in identifying patients who may respond favorably to PARPi treatment.
Specifications
| Target regions | Whole coding regions and intron/exon boundaries of BRCA1 and BRCA2 genes and ~24,000 genome-wide SNPs |
|---|---|
| Alterations detected | BRCA1/2 genes (SNVs/InDels) and Genomic Scar Score (GSS) |
| Sample type | FFPE tissue |
| DNA input | Optimal 100ng (minimum 50ng) |
| Data output per sample | 4 Gb |
| Sequencing type | PE150 |
| Sequencer | Illumina NextSeq 550Dx |
| TAT for library prep | 5 hours (>1 hour hands-on time) |
| TAT from sample to report | 3 days |
Materials for download
AmoyDx® HRD Focus Panel brochure
AmoyDx® HRD Focus Panel user manual
Products
Note: product availability depends on country - see product detail page.
| Details | Cat number & supplier | Size | Price |
| AmoyDx® HRD Focus Panel 8.06.0045 · Amoy Diagnostics | 8.06.0045 Amoy Diagnostics |
20 tests |
£17798.00
20 tests
view
|
| AmoyDx® HRD Focus Panel 8.06.0047 · Amoy Diagnostics | 8.06.0047 Amoy Diagnostics |
20 tests |
£17798.00
20 tests
view
|
| AmoyDx® HRD Complete Panel 8.06.0114 · Amoy Diagnostics | 8.06.0114 Amoy Diagnostics |
20 tests |
£17887.00
20 tests
view
|

