%20300x270.jpg)
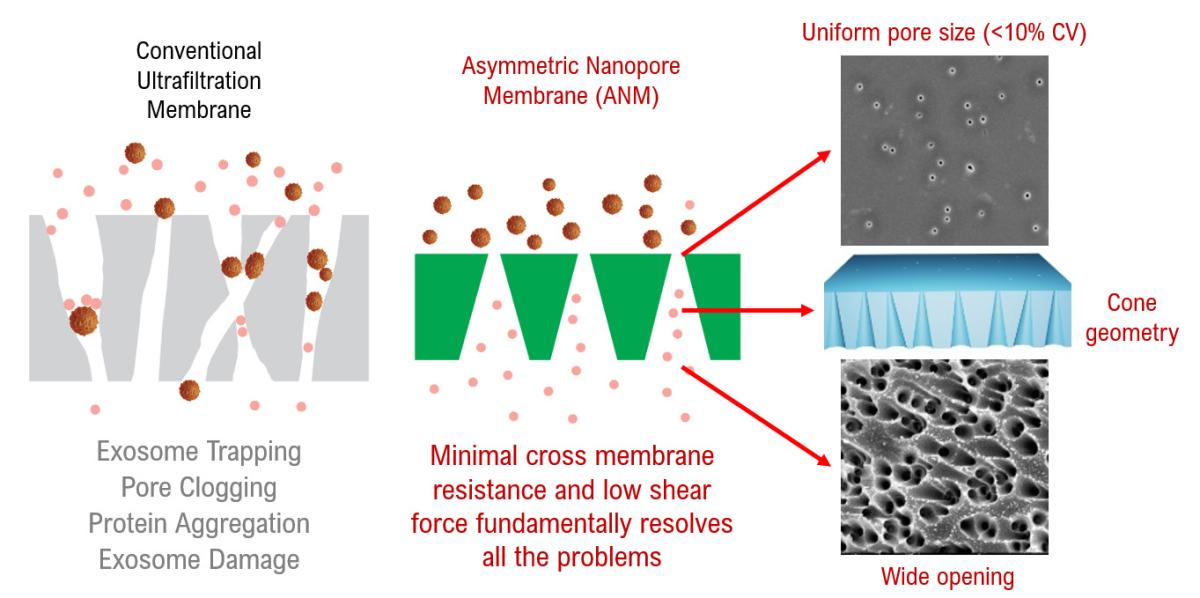
The NanoEx automated bio-nanoparticle isolation platform employ’s Aopia’s patented Asymmetric Nanopore Membrane (ANM) technology. It is a highly uniform and asymmetric pore design that prevents clogging, minimises shear forces, and ensures preservation of EV integrity. By combining high-efficiency bio-nanoparticle capture with advanced purifying action, ANM enables simultaneous concentration and enrichment, outperforming conventional ultrafiltration or tangential flow filtration approaches.
When assessing which EV isolation method best fits their needs, researchers must consider key factors such as removal of free protein, reduction in protein aggregation, specificity and purity of the isolation, breadth of sample compatibility, speed and hands-on time, and suitability for downstream processes. Here we will compare the NanoEX ANM technology with other methods on the market, such as tangential flow filtration (TFF), ultracentrifugation (UC), ultrafiltration (UF) and size exclusion chromatography (SEC), in the context of these important attributes.
Removal of free protein
![]() One of the biggest challenges in EV isolation is separating vesicles from the abundant free proteins present in biological fluids. NanoEX is designed specifically for this, with Aopia reporting >99.9% free-protein removal in their system. This is a major advantage for researchers conducting proteomic or functional assays where background protein can obscure results.
One of the biggest challenges in EV isolation is separating vesicles from the abundant free proteins present in biological fluids. NanoEX is designed specifically for this, with Aopia reporting >99.9% free-protein removal in their system. This is a major advantage for researchers conducting proteomic or functional assays where background protein can obscure results.
By comparison, TFF can remove a large portion of soluble protein but typically requires an additional polishing step, such as SEC, to achieve high purity. On its own, TFF-purified samples show a high concentration of remaining proteins (image 2). SEC itself is very effective at separating free protein by size, but cannot always distinguish EVs from similarly sized contaminants such as lipoproteins. UC and UF often leave significant protein contamination.
Reducing aggregation & preserving EV integrity
Another concern is the risk of protein aggregation and EV damage during isolation. UC, which relies on high centrifugal force, is well known to promote aggregation and can damage vesicles. UF can also introduce shear stress that affects sample quality.
NanoEX, by contrast, employs gentle, automated flow cycles designed to protect EV structure while removing impurities. SEC is also considered one of the gentlest approaches, though it sacrifices throughput and scalability. TFF is relatively mild compared to UC but still requires optimisation to avoid shear-related issues and is prone to aggregation. The below graph shows that filtrate from TFF can end up with more particles than the starting material, meaning protein aggregation has occured. In contrast, NanoEx EV isolation results in a lower number of particles than the starting material, but with a high recovery rate of 82%.
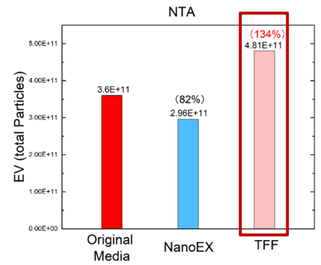
Data provided by Aopia
Specificity and purity
Purity is critical when researchers need to be confident they are working with EVs rather than co-isolated proteins or other nanoparticles. NanoEX’s asymmetric nanopores selectively intercept vesicles while flushing away proteins and other small molecules, offering high specificity in a single step.
SEC and TFF provide moderate purity but cannot fully discriminate between EVs and similarly sized particles. UC and UF are more likely to co-isolate non-EV material, unless combined with extra gradient or polishing steps. The below images show that NanoEx isolation results in much lower levels of total protein, with concentrated bands at CD9 and CD81, EV-specific markers, making it a much more specific method for EV isolation.
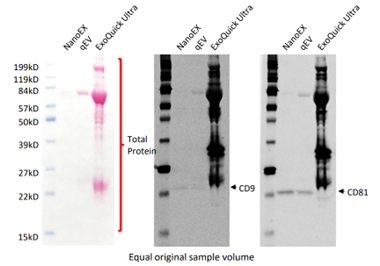
Data provided by Aopia
Compatibility across sample types
Different projects demand flexibility. Plasma, urine, cerebrospinal fluid, saliva, and cell culture medium all pose unique challenges. NanoEX is designed to handle this diversity and is also marketed for lipid nanoparticles (LNPs) and viral vectors, broadening its applications beyond EVs. EXODUS similarly highlights its broad compatibility with multiple biofluids.
TFF is highly versatile and remains the gold standard for large-scale processing, particularly in biomanufacturing. SEC, UC, and UF can be applied to a wide range of sample types but are often less efficient or less practical for higher volumes. The below image shows EVs from a range of starting material, specifically isolated with minimal protein contamination.
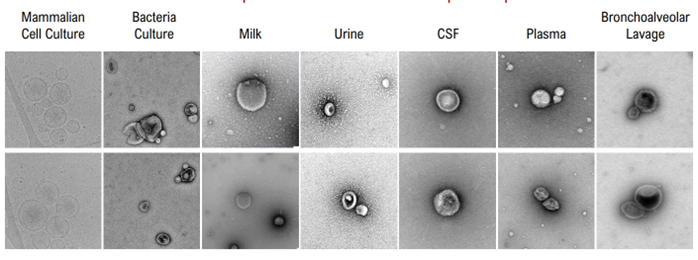
Data provided by Aopia
Hands-on time and clearer samples
One of the most practical considerations in any lab is how long a workflow takes. UC is notoriously time-consuming, requiring multiple long centrifugation steps. SEC is faster but still manual, while UF is quick but less reliable in terms of sample quality.
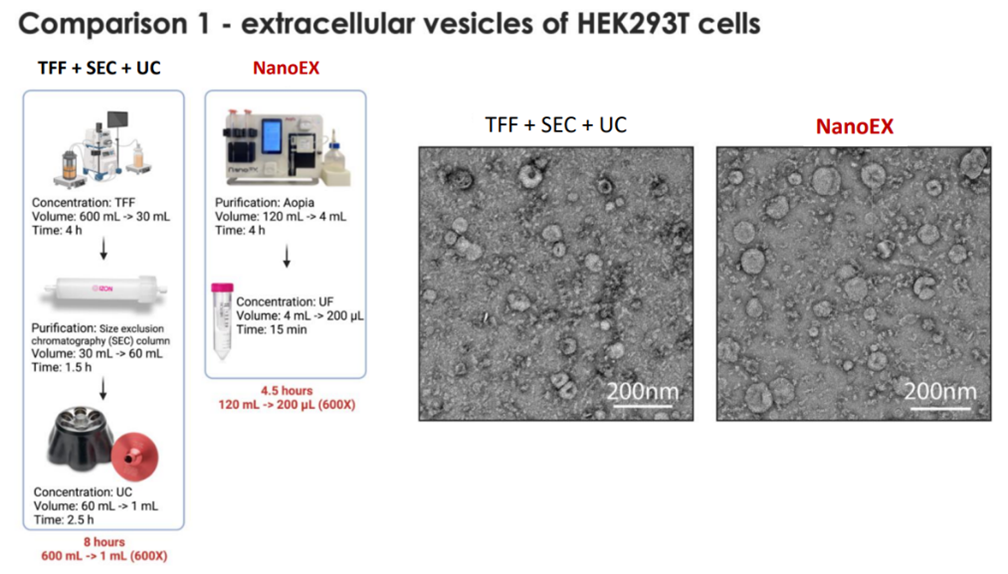
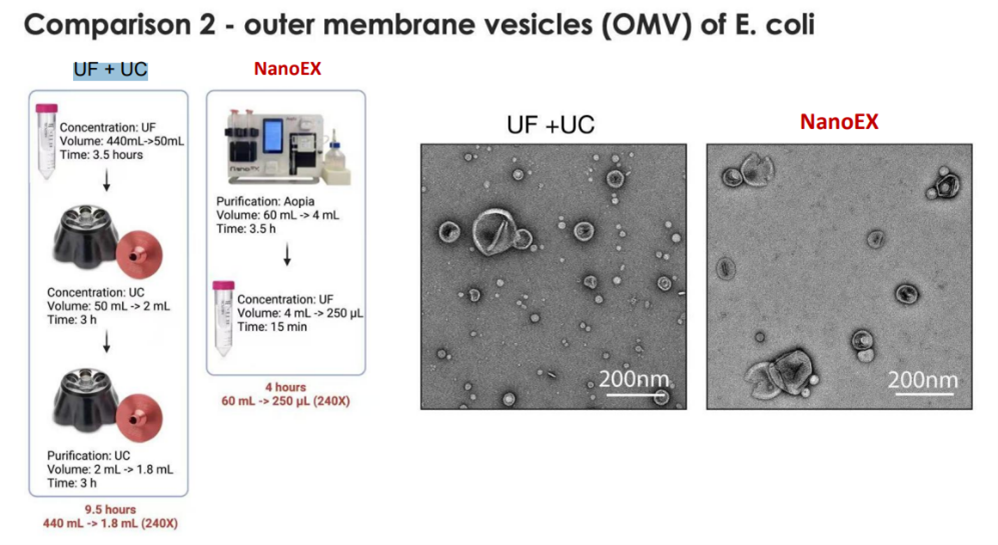
NanoEX automates the process, reducing hands-on time and variability while speeding up throughput. TFF is faster than UC and scales well, though it often requires combination with other steps. The below use cases highlight the speed of the automated workflow compared with other methods. In case 1, the NanoEx workflow took 4.5 hours, while the comparative TFF+SEC+UC workflow took 8 hours. In case 2, a UF+UC protocol took 9.5 hours,while the NanoEx run time for the same sample type was 4 hours.
For downstream applications such as proteomics, imaging, or functional assays, clarity matters. NanoEX is positioned as producing exceptionally clean samples, free of free protein and aggregates. This is evident in both cases below showing cleaner outputs.
|
|
|
|---|
Data provided by Aopia
How the NanoEx instrument compares
| Feature/metric | NanoEx | TFF | SEC | UF | UC |
|---|---|---|---|---|---|
| Free protein removal | High (>99.9%) | Moderate - needs SEC | Moderate-high | Moderate | Low-moderate |
| Aggregation risk | Low | Low-moderate | Very low | Moderate | High |
| Specificity/purity | High | Moderate | Moderate | Low-moderate | Low-moderate |
| Sample compatibility | Broad (fluids,LNPs, vectors) | Broad & scalable | Moderate | Moderate | Moderate |
| Hands-on time | Low (automated) | Moderate | Moderate | Low-moderate | High |
| Clarity | High | Moderate-high (with SEC) | Moderate-high | Moderate | Low-moderate |
| Recovery/yield | High (>80%) | High | Moderate | Moderate | Moderate-high |
| Scalability | Medium | Excellent | Low-moderate | Moderate | Low |
Contact our Aopia specialists to learn more about the NanoEx automated bio-nanoparticle isolation platform and how it can help your EV isolation workflows.