- In this section:
- SeraCare clinical genomics flyer
- SeraCare cancer solutions flyer
- SeraCare inherited disease solutions flyer
- SeraCare liquid Biopsy solutions flyer
- Tech note: Assuring quality for somatic tumour mutation profiling with Seraseq Solid Tumor Mutations Mix-1 (AF20)
- Tech note: Seraseq Circulating Tumor DNA-I Reference Materials for characterising, developing and validating plasma-based assays
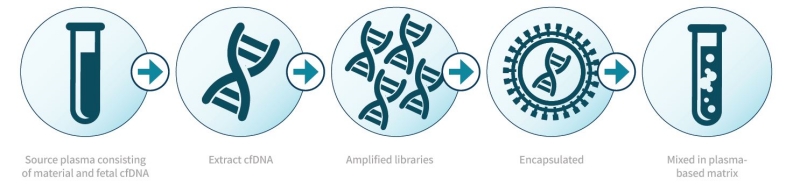
The Seraseq® NGS reference materials from SeraCare are highly multiplexed, patient-like standards that enable clinical research labs to properly develop, validate, monitor and standardise molecular next-generation sequencing (NGS) assays to support clinical evaluation and management of cancer and diseases.
With targeted NGS panels increasingly being used to discover causative variants for inherited disease, the use of cell-line materials and remnant patient samples, which only cover a small subset of target variants, is no longer informative enough for the spectrum of mutations that need to be covered. These standards provide consistent positive and negative controls across broad variant types, and are formatted as purified DNA (gDNA), purified circulating tumor DNA (ctDNA) in synthetic plasma-like material, as well as formalin-fixed, paraffin-embedded (FFPE) material.
Benefits of using Seraseq NGS reference standards
- Highly multiplexed, patient-like standards
- Expert-designed constructs with clinically relevant variants
- Assess common, rare and technically challenging variants
- Coverage across SNVs, insertions and deletions
- Enables proper development, validation, monitoring and standardisation of NGS assays
- Available as gDNA, ctDNA, synthetic plasma-like material or FFPE material
- Full-process standards for end-to-end monitoring of NGS workflow
- Manufactured in cGMP-compliant and ISO 13485-certified facilities
Seraseq innovative design process
These clinically relevant reference materials cover the following functional areas:
- Solid tumor profiling
- Liquid biopsy
- Hematological malignancy
- Tumor mutational burden
- Inherited diseases
Material available for download
SeraCare clinical genomics flyer
SeraCare cancer solutions flyer
SeraCare inherited disease solutions flyer
SeraCare liquid Biopsy solutions flyer
Tech note: Assuring quality for somatic tumour mutation profiling with Seraseq Solid Tumor Mutations Mix-1 (AF20)
Tech note: Seraseq Circulating Tumor DNA-I Reference Materials for characterising, developing and validating plasma-based assays
Below is a small selection of reference standards available. Please use our search for the full list of reference standards available.
Products
Note: product availability depends on country - see product detail page.
| Details | Cat number & supplier | Size | Price |
| SERASEQ LUNG & BRAIN CNV MIX, +12 COPIES 0710-0416 · LGC SeraCare | 0710-0416 LGC SeraCare |
1 each |
£936.00
1 each
view
|
| SERASEQ BREAST CNV MIX, +3 COPIES 0710-0411 · LGC SeraCare | 0710-0411 LGC SeraCare |
1 each |
£936.00
1 each
view
|
| SERASEQ SOLID TUMOR CNV MIX +3 COPIES 0710-2866 · LGC SeraCare | 0710-2866 LGC SeraCare |
1 each |
£1069.00
1 each
view
|
| SERASEQ CTDNA ESR1 MIX WT 0710-3564 · LGC SeraCare | 0710-3564 LGC SeraCare |
1 each |
£535.00
1 each
view
|
| SERASEQ® SOLID TUMOR FFPE DNA RM 0710-3634 · LGC SeraCare | 0710-3634 LGC SeraCare |
1 each |
£1361.00
1 each
view
|
| SERASEQ CTDNA ESR1 MUT MIX AF1% 0710-3565 · LGC SeraCare | 0710-3565 LGC SeraCare |
1 each |
£581.00
1 each
view
|
In this section
 SeraCare clinical genomics flyer
SeraCare clinical genomics flyer SeraCare cancer solutions flyer
SeraCare cancer solutions flyer SeraCare inherited disease solutions flyer
SeraCare inherited disease solutions flyer SeraCare liquid Biopsy solutions flyer
SeraCare liquid Biopsy solutions flyer Tech note: Assuring quality for somatic tumour mutation profiling with Seraseq Solid Tumor Mutations Mix-1 (AF20)
Tech note: Assuring quality for somatic tumour mutation profiling with Seraseq Solid Tumor Mutations Mix-1 (AF20) Tech note: Seraseq Circulating Tumor DNA-I Reference Materials for characterising, developing and validating plasma-based assays
Tech note: Seraseq Circulating Tumor DNA-I Reference Materials for characterising, developing and validating plasma-based assays