We provide researchers with fresh human leukopaks for delivery on the same or next day after collection, in partnership with Research Donors.
Our fresh human leukopaks are a source of highly concentrated peripheral blood mononuclear cells (PBMC) from a single donor, with a range of sizes available depending on your requirements. Collections are tailored to each researchers' specific needs, so the choice of volume, shipment temperature and blood group is yours.
See what researchers say about our leukapheresis service
Benefits of using Research Donors fresh human leukopaks
- 10 billion total nucleated cells per donor on average from a full leukopak
- Delivery on the same or next day after collection (location dependent)
- Fresh leukopaks collected to your exact research requirements
- High-viability PBMCs with minimal degradation
- Fresh leukopak collection available 4 days per week
- Comprehensive donor information provided, including full blood counts
- Collected at an HTA-licensed, ISO 9001 2015 certified facility
- Samples fully consented including for genetic analysis, animal models and commercial research purposes
- Large donor pool
- Available in full, half and quarter bags or 5-15 ml (500 million TNCs) sizes
- Donors pre-screened for HIV, HBV, HCV, Syphilis, HTLV I and HTLV II
Who are Research Donors?
Research Donors is an HTA-licensed clinic, based in London, dedicated to the collection and processing of human blood and fresh leukopaks for research purposes. Research Donors is ISO 9001 2015 certified with Research Ethics (REC) approval as a Research Tissue bank, and participates in the UK NEQAS QA scheme.
Leukopak validation data
|
|
|
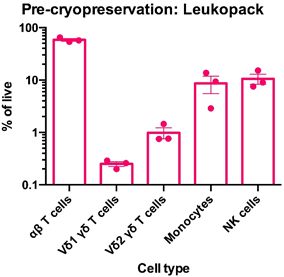
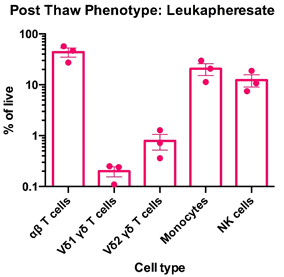
Left: Flow cytometry was used to assess the expression of CD3, TCR α/β, TCR Vδ1, TCR Vδ2, CD14 and CD56 within PBMC freshly-isolated from leukoapheresates from three healthy donors. The data for αβT cells (CD3+TCR α/β +), Vδ1 yδT cells (CD3+TCR Vδ1+), Vδ2 yδT cells (CD3+TCR Vδ2+), monocytes (CD3-CD14+) and NK cells (CD3-CD56) is shown as mean percentage + SEM. Right: Flow cytometry was used to assess the expression of CD3, TCR α/β, TCR Vδ1, TCR Vδ2, CD14 and CD56 within PBMC immediately post cryorecovery in 10% DMSO from leukoapheresates from three healthy donors. The data for αβT cells (CD3+TCR α/β +), Vδ1 yδT cells (CD3+TCR Vδ1+), Vδ2 yδT cells (CD3+TCR Vδ2+), monocytes (CD3-CD14+) and NK cells (CD3-CD56) is shown as mean percentage + SEM.
Data courtesy of Jonathan Fisher et al, UCL Great Ormond Street Institute of Child Health, London, UK.
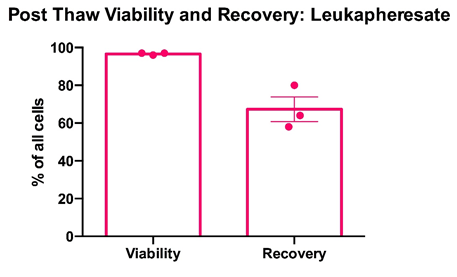
PBMCs were cryopreserved in 10% DMSO and post-thaw viability was determined via trypan blue exclusion. Cell counts pre- and post-cryopreservation were used to calculate the percentage recovery. Mean percentage + SEM and individual values are shown for PBMCs from leukoapheresates from three healthy donors. Mean cell recovery after cryopreservation was 67% for leukapheresate samples.
Data courtesy of Jonathan Fisher et al, UCL Great Ormond Street Institute of Child Health, London, UK.
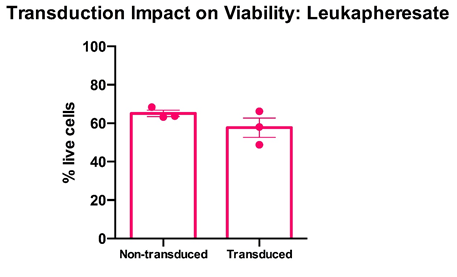
Cryopreserved PBMC from leukoapheresates from three healthy donors were thawed, rested overnight in complete media (RPMI1640 supplemented with L- Glutamine and 10% FCS) and stimulated with a translationally relevant T cell expansion cocktail; specifically, PBMC received a single does of TCR stimulus post-thaw and were cultured in complete media containing 100 IU/mL IL-2. The expansion was supplemented with fresh IL-2 containing media every 2-3 days. T cell viability was assessed using flow cytometry after 12 days of stimulation for cell viability. Day 12 overall cell viability was consistently >60% for all samples, with marginal impact on post-transduction. Mean percentage + SEM and individual values are shown for n=3.
Data courtesy of Jonathan Fisher et al, UCL Great Ormond Street Institute of Child Health, London, UK.
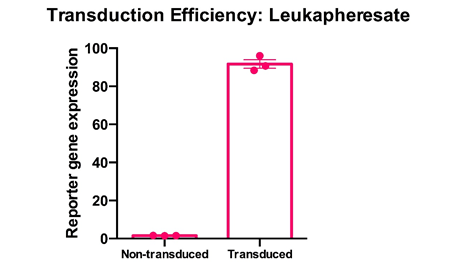
Cryopreserved PBMC from donor matched leukapheresates from three healthy donors were thawed, rested overnight in complete media (RPMI1640 supplemented with L- Glutamine and 10% FCS) and stimulated with a translationally relevant T cell expansion cocktail; specifically, PBMC received a single does of TCR stimulus post-thaw and were cultured in complete media containing 100 IU/mL IL-2. The expansion was supplemented with fresh IL-2 containing media every 2-3 days. T cell transducibility was assessed by viral transduction using a translationally-relevant lentiviral vector and protocol. T cell transduction efficiency was assessed on day 12 of expansion. T cells of donor matched leukapheresate origin were highly and efficiently transducible with a lentiviral multiplicity of infection of 4, with a mean efficiency of >90%. Mean percentage + SEM and individual values are shown for n=3.
Data courtesy of Jonathan Fisher et al, UCL Great Ormond Street Institute of Child Health, London, UK.
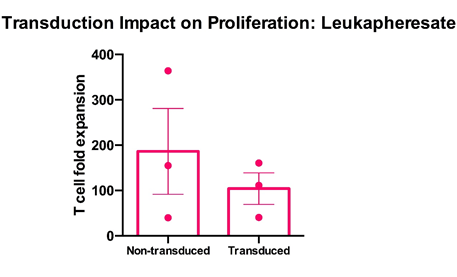
Cryopreserved PBMC from three healthy donors were thawed, rested overnight in complete media (RPMI1640 supplemented with L- Glutamine and 10% FCS) and stimulated with a translationally relevant T cell expansion cocktail; specifically, PBMC received a single does of TCR stimulus post-thaw and were cultured in complete media containing 100 IU/mL IL-2. The expansion was supplemented with fresh IL-2 containing media every 2-3 days. T cell expansion was assessed using flow cytometry and counting beads after 12 days of stimulation for cell viability. Non-transduced leukapheresate T cells expanded at a mean >180-fold, whilst transduced T cells expanded to a similar extent at a mean 100-fold. Mean percentage + SEM and individual values are shown for n=3.
Data courtesy of Jonathan Fisher et al, UCL Great Ormond Street Institute of Child Health, London, UK.
Applications
- Immunology research
- Cell therapy research e.g. CAR-T
- Toxicology studies
- High-throughput drug screening
Contact our blood specialists
To request a quote or find out more, please contact our blood specialists.
These products are for use in Biomedical Research only. They are not to be used for any form of human treatment, or for any purpose that is prohibited by law, including any use for human reproductive cloning. It is your responsibility to ensure that you have necessary licensing and ethics approvals in place for the use and storage of any human tissue related material supplied by Cambridge Bioscience.