ClearColi® cells from Lucigen are the first commercially available electrocompetent cells with a genetically modified lipopolysaccharide (LPS) that does not trigger an endotoxic response in mammalian cells. Instead of removing LPS contamination from protein or plasmid DNA preparations after production using downstream applications, the ClearColi cells effectively eliminate LPS at the source, producing functionally clean recombinant proteins and plasmids.
Benefits of using the ClearColi BL21(DE3) cells
• No endotoxic response triggered in mammalian cells
• False positives reduced in cytokine assays
• Useful for membrane and lipid binding protein production
• Ideal for immunogenicity testing, toxicity assays and therapeutic protein drug discovery
| Method | Disadvantages |
|---|---|
| Ultrafiltration | • Only useful for small proteins • Endotoxin monomers may permeate the membrane due to endotoxin/protein interaction • Inefficient for proteins which can be damaged by physical forces |
| Activated carbon | • Adsorbing activity for both endotoxin and protein |
| Surfactants | • Expensive • May affect bioactivity of protein • Difficult to completely remove • Removal may lead to product loss |
| Anion-exchange chromatography | • High adsorbtion of both endotoxin and acidic protein • No selectivity to adsorb endotoxin |
| Histamine- and histidine-immobilised sepharose | • Removing capacity dependent on the ionic strength • Biological activity of histamine |
| Polymyxin B-immobilised sepharose | • Protein losses due to the ionic interaction between polymyxin B and protein • Polymixin B is physiologically active |
Comparison of protein expression in ClearColi BL21(DE3) and Lucigen's E. Cloni® EXPRESS BL21(DE3) competent cells
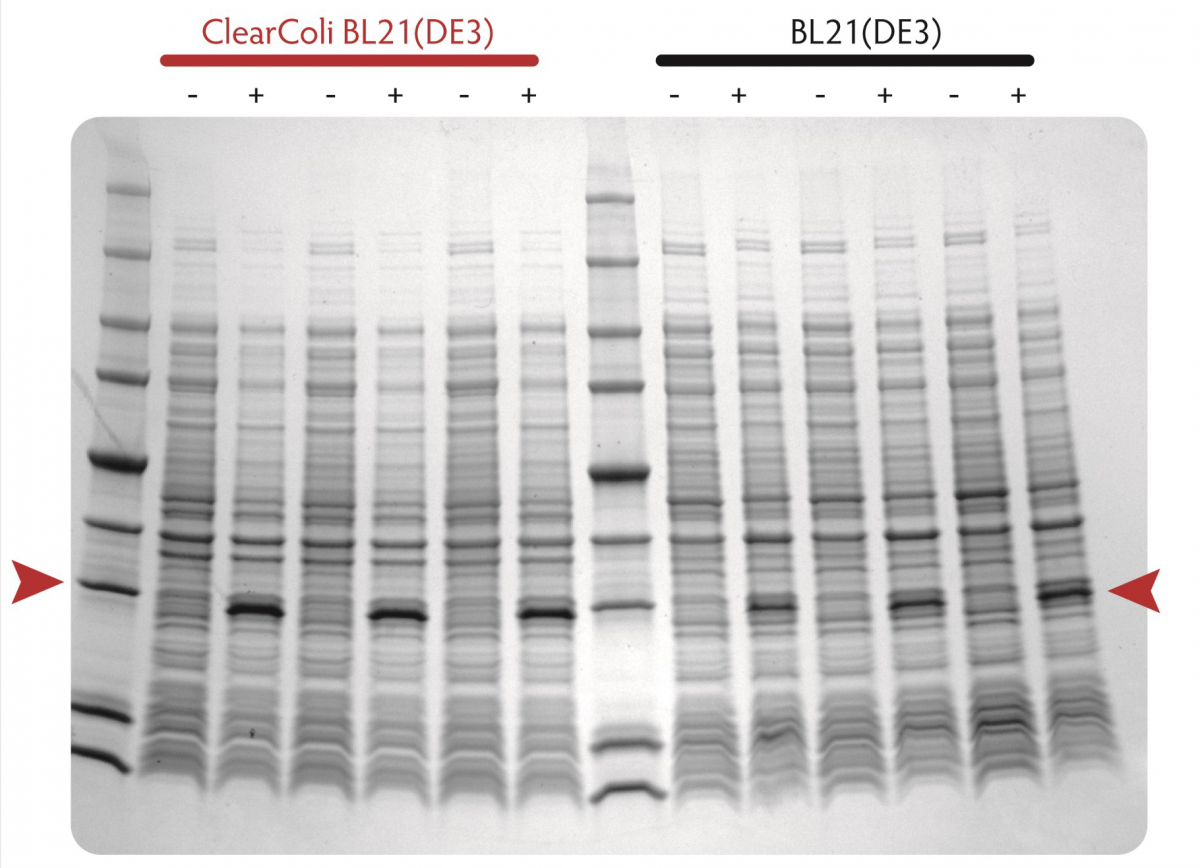
Cells containing a T7 expression plasmid harbouring a gene encoding the human apolipoprotein A1 (ApoA1) were grown in LB Miller medium at 37°C. When cultures reached OD600 of 0.6 to 0.8, expression was induced by the addition of 0.4 mM IPTG and incubation was continued for three hours. Equivalent numbers of uninduced (-) and induced (+) cells were lysed by heating in Laemmli buffer and samples were analysed by SDS-PAGE on a 4% - 20% polyacrylamide gradient gel.
Comparison of endotoxin units as measured by the LAL assay using nickel-column purified recombinant ApoA1 and HSP protein expressed in ClearColi BL21(DE3) and E. cloni EXPRESS BL21(DE3) competent cells
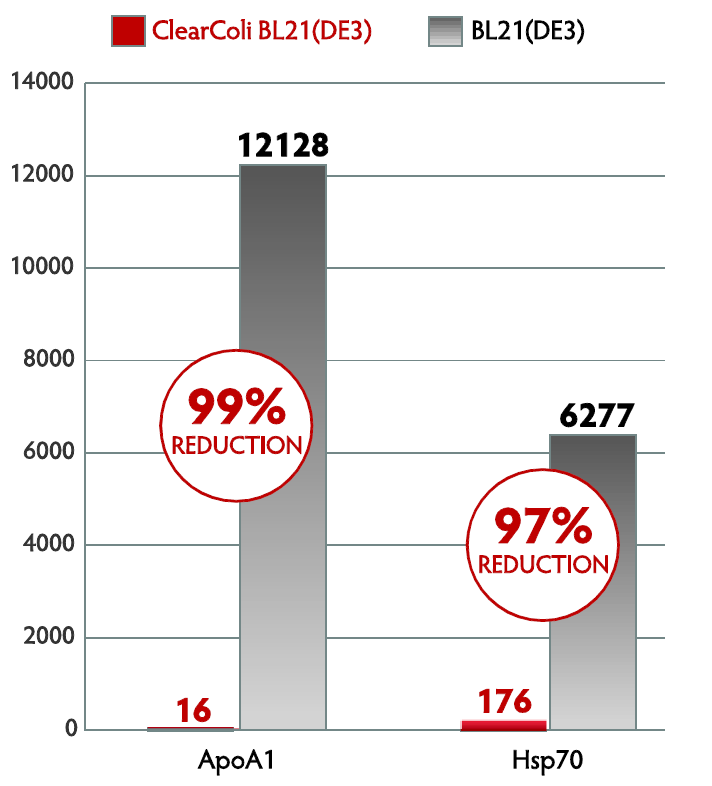
Protein expressed from ClearColi demonstrates significant reduction in EU/mg without endotoxin removal steps.
Material available for download
ClearColi BL21(DE3) user manual
Application note: Endotoxin-free protein production—ClearColi technology
Application note: Endotoxin-Free ClearColi BL21 (DE3) for protein expression
ClearColi citations
ClearColi licensing restrictions
Please note certain licensing restrictions apply to the ClearColi products for non-commercial research purposes only. A separate license is required for any commercial use. Find out more.
Products
Note: product availability depends on country - see product detail page.
| Details | Cat number & supplier | Size | Price |
| ClearColi BL21(DE3) Electrocompetent Cells 60810-1 · Lucigen | 60810-1 Lucigen |
12 reactions (duos) |
POA
12 reactions (duos)
view
|
| ClearColi BL21(DE3) Electrocompetent Cells 60810-2 · Lucigen | 60810-2 Lucigen |
24 reactions (duos) |
POA
24 reactions (duos)
view
|

